
INSPECTIFAI
Taking Visual Inspection to New Heights
Next-level quality control for greater drug and patient safety in pharma. We believe in the power of data and their ability to radically optimize visual inspection in the pharmaceutical industry.
-89% ⌀
False Reject Rate
+38% ⌀
Detection Rate
1 Day
On-Site Implementation
Boost your Visual Inspection Performance
We have launched our GxP compliant AI solution with full commitment to helping companies improve their drug quality while increasing efficiency, saving costs, and creating a positive impact on sustainability. Being regulatory-compliant, INSPECTIFAI EMBRAICE functions globally, as machine-agnostic solution that can be implemented on a wide range of vision applications and Automated Visual Inspection machines – for a centralized approach!
Minimize requalification efforts
Achieve higher machine throughput
Speed up recipe optimizations
Minimize recipe landscape
Shorten time-2-inspection for new products
Positive impact on sustainability
Our Partners & Memberships
INSPECTIFAI's network of partners and memberships supports the implementation as well as the continuous development of our solution and customer benefits.





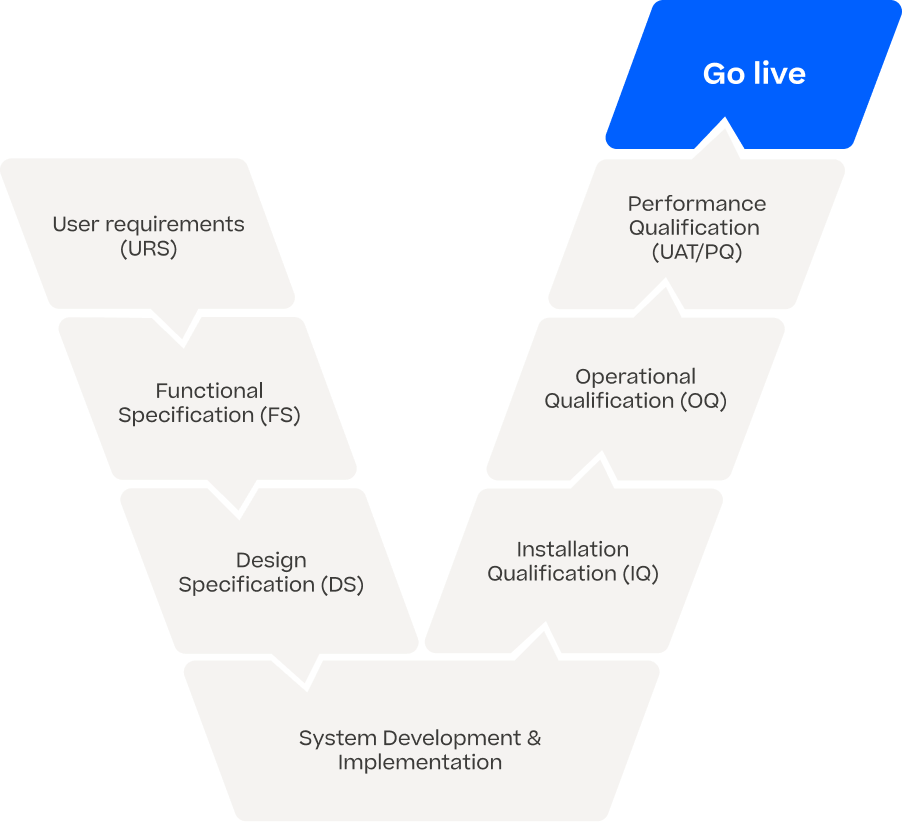
Regulatory Compliance
With our end-to-end solution, we ensure regulatory-compliant AI on a wide variety of vision applications and Automated Visual Inspection systems without any significant changes or requalification efforts on your machine. We work GxP compliant in developing state-of-the-art software and bringing INSPECTIFAI EMBRAICE onto your vision system. Our documentation and validation package is part of our delivery and makes AI applicable in the regulated industry.
